Manufacturer Good Price Aniline CAS:62-53-3
Description
Aniline is an important chemical raw material, the production of more important products up to 300 kinds, mainly used in MDI, dye industry, medicine, rubber vulcanization promoters, such as p-aminobenzene sulfonic acid in dye industry, medicine industry, N-acetanilide, etc. It's also used to make resins and paints. In 2008, the consumption of aniline was about 360,000 tons, and the demand is expected to be about 870,000 tons in 2012. Chemicalbook has a production capacity of 1.37 million tons, with an excess capacity of nearly 500,000 tons. Aniline is very toxic to the blood and nerves, and can be absorbed through the skin or cause poisoning through the respiratory tract. There are two main methods to produce aniline in industry: 1. Aniline is prepared by hydrogenation of nitrobenzene catalyzed by active copper. This method can be used for continuous production without pollution. 2, chlorobenzene reacts with ammonia at high temperature in the presence of copper oxide catalyst.
Synonyms
ai3-03053;amino-benzen;Aminophen;Anilin;anilin(czech);Anilina;BENZENEAMINE;BENZENAMIN.
Applications of Aniline
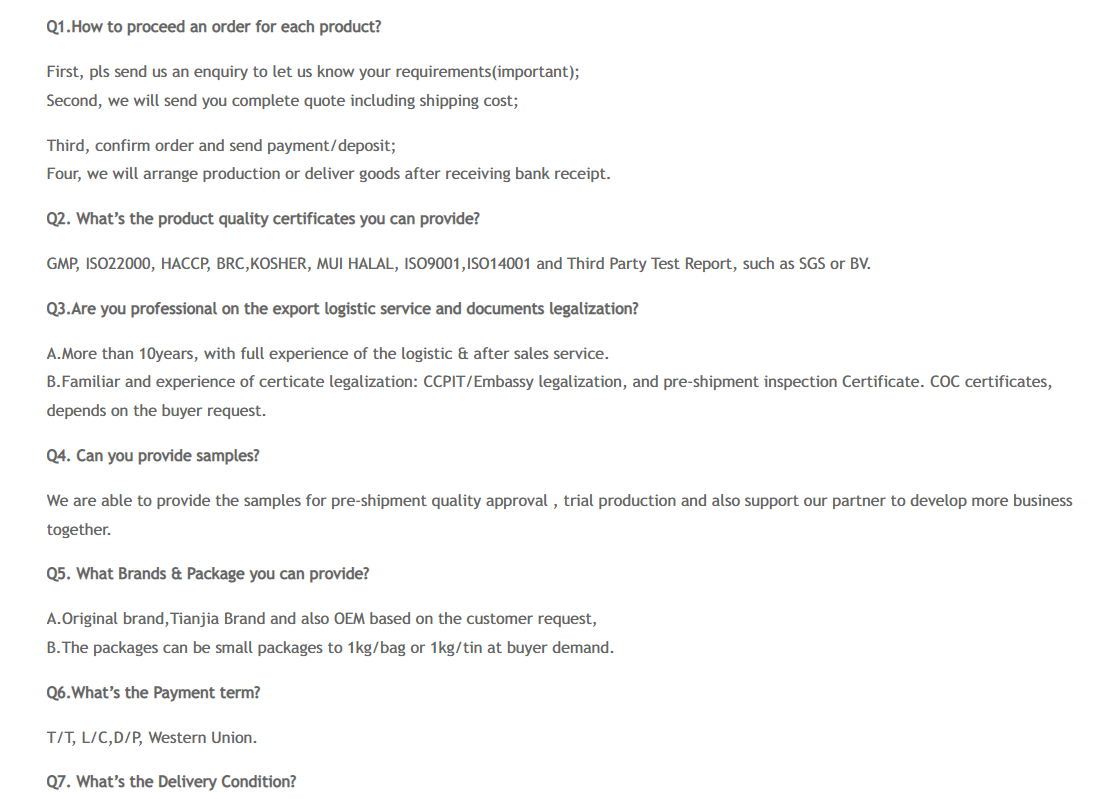
1. Aniline is one of the most important intermediates in the dye industry, and it is also the main raw material for medicine, rubber promoters and anti-aging agents. It can also be used to make spices, varnishes and explosives, etc. Aniline is used in the manufacture of dyes, medicines, resins, varnishes, perfumes, Chemicalbook vulcanized rubber and even solvents. Hazardous and harmful substances affecting the early life stages of Marine animals. According to the US Environmental Protection Agency (EPA), Environmental and food contaminants, Drinking water contaminants Candidate Compound 3(CCL3).
2. Aniline is an important raw material, the production of pesticides can be derived from aniline, alkyl aniline, N - alkyl aniline adjacent nitro aniline, o-phenylendiamine, phenylhydrazine, cyclohexylamine etc., can be used as a fungicide against rust sodium, the seed spirit, amine methyl Chemicalbook sterilization, sterilization amine, carbendazim, its spirit, benomyl, triazophos insecticide, pyridazine sulfur phosphorus, quetiapine phosphorus, Intermediates of herbicides alachlor, acetochlor, butachlor, cycloazinone, imidazole quinolinic acid, etc.
3. Aniline is an important intermediate. More than 300 kinds of important products are produced from aniline. There are about 80 aniline manufacturers in the world, the total annual production capacity has exceeded 2.7 million t/a, the output of about 2.3 million t; The main consumption area is MDI, which accounts for 84% of the total consumption of aniline in 2000. In our country, aniline is mainly consumed in the MDI, dye industry, rubber additive, medicine, pesticide and organic intermediates. The consumption of aniline in 2000 is 185,000 t, and the shortage of production needs to be solved by import. Aniline intermediates and dye products are: 2, 6-diethyl aniline N-acetaniline, p-butyl aniline, o-phenylenediamine, diphenylenediamine, diazo-aminobenzene, 4,4' -diaminotriphenylmethane, 4,4' diaminodiphenylcyclohexyl methane,N, N-dimethylaniline, N-diethylaniline,N, n-diethylaniline, p-acetamide phenol, p-aminoacetophenone,4 ,4' -diethylaminophenone,4- (p-aminophenine) butyric acid, p-nitroaniline, N-nitrodianiline, β-acetaniline, 1, 4-diphenylaminourea, 2-phenylindole, p-benzaniline, N-formylaniline, n-benzoylaniline, n-acetaniline, 2,4, 6-trichloraniline, p-chemicalbook iodoaniline , 1 - aniline - 3 - methyl - 5 - pyrazole ketones, hydroquinone, dicyclohexyl amine, 2 - (N - methyl aniline) acrylic nitrile, 3 - (N - diethyl aniline) acrylic nitrile, 2 - (N - diethyl aniline) ethanol, p-aminoazobenzene, phenylhydrazine, phenyl urea single, double phenyl urea, of sulfur cyano aniline, 4, 4 'diphenyl methane diisocyanate, phenyl methyl many many times more Cyanate ester, 4-amino-acetanilide, N-methyl-N - (β-hydroxyethyl) aniline, n-methyl-N (β-chloroethyl) aniline,N, N-dimethyl-p-phenylenediamine,N,N,N',N' -tetramethyl-p-phenylenediamine,N, n-diethyl-p-phenylenediamine, 4,4' -methylenediamine (N, n-diethyl-p-phenylenediamine, phenylthiourea, diphenylenediamide, p-amino Benzene sulfonic acid, 4, 4 'diamino diphenyl methane benzoquinone, N, N - against ethanol base aniline, acetyl acetanilide, aminophenol, N, N - methyl - ethyl benzyl aniline formyl aniline, N - methyl acetanilide, the bromine acetanilide, double (to amino cyclohexyl) methane, phenylhydrazone diphenyl kappa hydrazone and acetophenone phenylhydrazone - 2, 4 - disulfonic acid, aniline, p-aminoazobenzene - 4 'sulfonic acid, phenylhydrazine -4- sulfonic acid, thioacetanilide, 2-methylindole, 2, 3-dimethylindole, N-methyl-2-phenylindole.
4, used as an analytical reagent, also used in the synthesis of dyes, resins, false paints and spices.
5.Used as a weak base, it can precipitate easily hydrolyzed salts of trivalent and tetravalent elements (Fe3+, Al3+, Cr3+) in the form of hydroxide, so as to separate them from the salts of divalent elements (Mn2+) that are difficult to hydrolyze. In picrystal analysis, to examine elements (Cu, Mg, Ni, Co, Zn, Cd, Mo, W, V) that are capable of forming Chemicalbook thiocyanate complex anions or other anions that can be precipitated by aniline. Test for halogen, chromate, vanadate, nitrite, and carboxylic acid. Solvents. Organic synthesis, dye manufacturing.



Specification of Aniline
|
Compound |
Specification |
|
Appearance |
Colorless,oily,yellowish,transparent liquid, tending to be darker after being stocked. |
|
Purity % ≥ |
99.8 |
|
Nitrobenzene % ≤ |
0.002 |
|
High Boilers % ≤ |
0.01 |
|
Low Boilers % ≤ |
0.008 |
|
Moisture % ≤ |
0.1 |
Packing of Aniline


200kg/drum
Storage: Preserve in well-closed, light-resistant, and protect from moisture.
FAQ